Reproduction
Hermit crabs are gonochoristic, meaning the sexes are separate. In both males and females, the reproductive organs, testes or ovaries, are housed within the abdomen. These reproductive organs are large, along with the hepatopancreas making up most of the abdominal mass. Hermit crabs reproduce by copulation. Lacking flagella, the sperm are immobile and for this reason are packaged into structures known as spermatophores (Tudge 1991). The spermatophore consists of a base or pedestal, stalk and the sperm filled ampulla or head. The spermatophore of Dardanus megistos is very short and simple in shape, strikingly different in form from that of D. lagopodes, being more similar to that typifying the species of Clibanarius (Tudge 1991). Sperm is produced in the testes, packaged into spermatophores in its passage through the vas deferens where it is also stored distally (Tirelli et al. 2010). Spermatophores are then transferred to females via ovular setal-lined openings of the coxae (basal/proximal segment) of the fifth pereopod called gonopores (Turra 2004). Females also have gonopores which receive the spermatophore, but are located on the coxae of the third pereopods (Turra 2004).
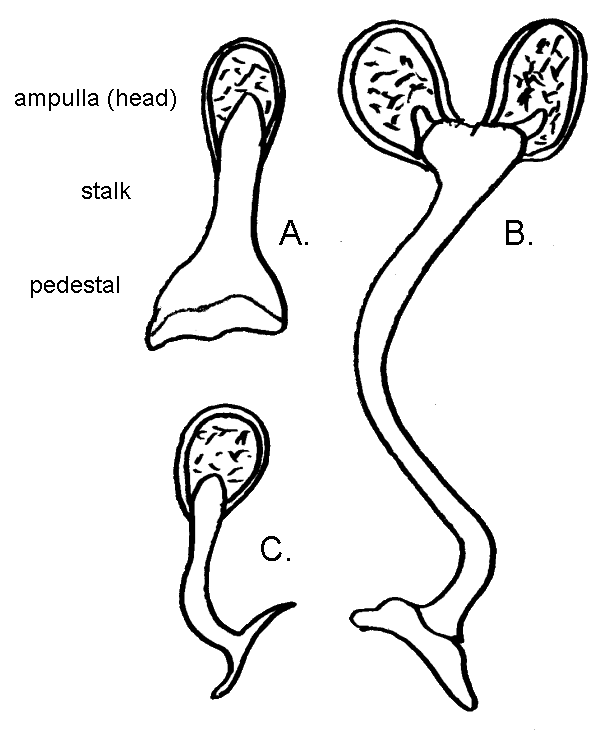 |
| The spermatophore of A. Dardanus megistos is much simpler than that of B. Dardanus lagopodes and is instead more similar to a morphology typical of C. Clibanarius. The immobile sperm is carried within the ampulla. Illustration by Storm Martin 2012, adapted with modification from Tudge 1991 |
Male hermit crabs actively track sexually reproductive females using chemosensory cues but may also come across females via chance encounter (Hazlett 1972). After encountering a female, the male holds onto the aperture of her shell with the minor cheliped and will drag her with him for as long as several days until she becomes ready to spawn (Hazlett 1972). This behaviour is described as female guarding and during this period the male may need to defend his right to mate from other competing males (Hazlett 1972). While varying with species and depending on local population structure, these antagonistic encounters with competitor males can be quite common (Hazlett 1996). The attacking male attempts to pull or dislodge the guarding male, who at first waves his large cheliped and walking legs to intimidate and discourage his rival and then, maintaining a position between his female and the attacker, pushes his competitor away and if possible onto his back (Hazlett 1972). In more serious fights the defender may poke at the aggressors eye stalks and sensory appendages with the pointed dactyl of its walking legs (Hazlett 1972). Sometimes a male will be forced to drop his guarded female in order to battle with an aggressor and in these situations the female often escapes (Hazlett 1972).
Hermit crab reproduction can be described as a male mate choice system as males actively search for, assess and select females to mate with (Wada et al. 2010). A male may reject his guarded female when encountering a higher quality mate (Wada et al. 2010) and some species have even been known to guard two females simultaneously (Hazlett 1972). This behaviour hasn’t been recorded from Dardanus megistos, though it is not unreasonable to suggest, given the extreme sizes males may reach. Larger females are considered higher quality mates due to their increased fecundity and the largest males always choose the largest female possible (Wada et al. 2010). Smaller males need to choose more carefully; while a larger female will give more offspring, a small guarding male is more likely to be challenged and defeated by larger competitors if he has a high quality mate (Wada et al. 2010). His chances of securing copulation also decrease the longer the guarding period, whereas a large male can successfully fend off several competitors over multiple days (Wada et al. 2010). Females do not exhibit mate choice, though are likely to resist and escape a male smaller than themselves (Wada et al. 2010). The quality and internal volume of the female’s shell can limit or even prevent production of eggs and so shell selection is another component of female quality (Terrosi et al. 2010).
While guarding a female the male displays courting behaviour. This behaviour varies between species but in diogenid hermit crabs generally consists of rocking and rotating the female shell as well as probing the aperture, stroking, grasping or tapping her pereopods or tapping her shell either internally or externally (Hazlett 1972). The male may rock and tap the female shell violently if she fully withdraws so as to encourage her back out, particularly as copulation draws near (Hazlett 1972). The female signals to the male that she is ready to copulate by movements of her chelipeds and antennae in the region of the male’s eyestalks and antennules (Hazlett 1972). The pair then copulate; both partially extend from their shells, they intertwine their walking legs and the male positions his gonopores of the fifth pereopods against her gonopores of the third pereopods for transfer of the spermatophores (Hazlett 1972). The male may attempt multiple copulations while guarding the female of which several may be successful (Hazlett 1972). After copulation the successful male continues to hold the female, only releasing her and moving on after she has extracted her newly fertilised eggs (Hazlett 1972). The eggs are held against the abdomen with the pleopods, under the protection of the gastropod shell (Hazlett 1972). Females may mate with more than one male and therefore may be carrying eggs from multiple fathers at a time (Hazlett 1972).
There is evidence of the capability for sex change amongst at least some species of hermit crab (Hazlett 1972, Turra 2004). The discovery of intersex individuals, those that display reproductive characteristics of both male and female, has been used to support these sex change hypotheses (Turra 2004). Intersex individuals are considered to be in the process of changing sexes, female to male, and in hermit crabs are most easily identified by the presence of gonopores on both pereopods 3 (female) and 5 (male) (Turra 2004). In some species, where the majority of smaller individuals are females and those of larger size classes are mostly male (Hazlett 1972), a relatively high prevalence of intersex individuals at intermediate sizes provides evidence for sex change. Intersex individuals may still achieve successful copulations, acting as males (Turra 2004). Sex change from female to male as size increases may seem an odd reproductive strategy, considering that female fecundity has a direct positive relation with size. However, larger individuals are often constrained by a limited selection of shells. A small or poor quality shell limits female reproductive output and so smaller females are more likely to achieve their full reproductive potential (Terrosi et al. 2010). Conversely, large males have greater chances of securing mates and thereby maximising their reproductive output. Sex change has only been demonstrated in some hermit crabs and not yet in Dardanus megistos. Further, even in those species which display intersex, intersex individuals are never prevalent and not all individuals undergo sex change (Turra 2004).
|